What Do You (Mis)Understand About pH?
By Jim Smith
Understanding pH can be difficult, yet a big part of what we do in cleaning is chemistry. Knowing about pH means you can either avoid a lot of problems, possibly fix them, or at least explain them.
Degreed chemists in our industry will often comment that we have drawn the wrong conclusions about what we know. Don’t we understand at least the basics of what is essential? Probably not.
pH misinformation
It is not that we were intentionally deceived by anyone; we’ve just been misinformed. Here are the little lies we have been taught:
- pH measures the strength of acids and alkalines.
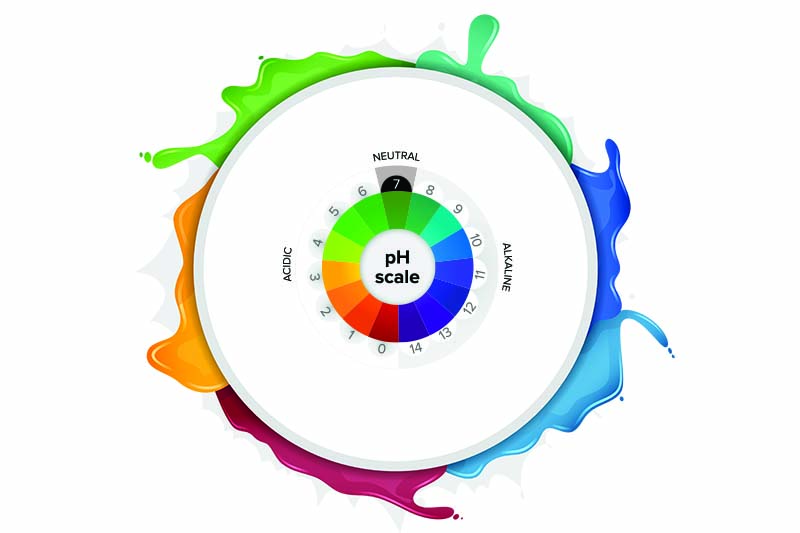
- The pH scale goes from zero to 14.
- Values in the pH range from zero to seven are acids; those in the seven to 14 range are alkalines; and seven itself is neutral.
Here’s what went wrong: We added our powers of reasoning to these half-truths, which resulted in further miseducation. Therefore, we are wrong when we say things like:
- A pH of 2 should be neutralized with a pH of 12.
- A pH of 1 or 13 is very powerful.
- pH is the measurement of acidity and alkalinity.
It appears we have been taught the wrong pHacts. Going back to what we learned, we were taught that all pH measurements measure acids and alkalinity. Therefore, educators thought it would be okay to teach the pH scale alone. However, the pOH scale measures alkalinity.
Zero to 14
Under ideal conditions, the pH + pOH of water can equal 14. These scales really exist for convenience. Try googling “pH negative one” and “pH 15” and you might be surprised to find that they exist.
So, what is the point of emphasizing the scale? And is seven really neutral? Again, only under ideal conditions. However, most pH meters do not measure pH as it is given in its definition as pH = -log10[H+]. Notice that temperature is not given in this equation. Therefore, most pH meters adjust the real pH so that the pH + pOH equals 14 just to make it easy to understand.
Many might wonder why pH is not the measurement of acidity and alkalinity. Well, it’s close. In fact, it can be a measurement of pH. The pH of a solution is controlled by buffering and ionic strength. Thus, one can have a weak pH of 13 or a powerful pH of 7. Moreover, whether they react with anything can depend upon other factors aside from whether the values are strong or weak.
Using pH knowledge
Now that everything you were taught about pH turns out to be a myth and all of your conclusions are wrong, where do we go next?
Should we just stop teaching pH? Would that solve the problem? No. We would be better off knowing the misinformation than no science at all.
Should we require a PhD in chemistry before cleaning carpet? I think that those who have a PhD would say no (and carpet cleaners would scream it).
Are there other facts about pH that we missed that would give us real science we could understand and use?
The answer to the last question is a big “yes.” Swimming pool cleaners deal with chemistry, and in particular, pH. What do they know that we don’t, and how do they use their knowledge? They know pH meters and how to read charts. This is all our industry’s technicians really need to know, too.
Electronic pH meters can tell us what kind of product was last used to clean the carpet, which allows cleaners to avoid assuming the liabilities of what would have been the previous cleaner’s lawsuit.
By knowing the pH of a spot or stain, one can know its source and the best way to remove it. A contrast in pH between the spot and the surrounding carpet tells us that the spot is due to a water-based problem and that a water-based spotter with a contrasting pH will be the most effective spotter to use. When there is no contrast in pH, we know that a dry solvent is the best spotter in that case.
These pH meters can also tell us when color loss is caused by chemicals or if it is coming from the atmosphere or sun fading. Once again, a chemical that causes color loss will leave a different pH compared to the nonaffected area. When it is a natural colorloss, there will be no contrast in pH.
Where does one gain this knowledge about pH? Some instructors teach pH meters, and some do not. Aside from that, there is YouTube. Some manufacturers of pH meters have training videos that show how to use their products on carpet.
Trust me, if your customer sees you using a pH meter, they will be impressed. Furthermore, a photo record of your readings from a cleaning job can eliminate a lot of legal calamities. This is always better than revealing that all you know about pH is junk science.
James “Jim” B. Smith is an IICRC-approved instructor and a senior practicing inspector and part of the voting consensus of the IICRC S100 cleaning standard. His educational studies come from Texas A&M University and the University of Houston. He has been in the cleaning industry since 1975. For more information, visit his website atcarpetinspector.com/jbs, call 972-334-0533, or email [email protected].












