Environmental Testing in Restoration and Remediation Projects

By Slade Smith
The current events surrounding the COVID-19 global pandemic have brought to the public’s attention the concepts of “testing” and “monitoring” for evaluating one’s health relative to the general public. I think many of us know what a swab is by now, and some of you might have actually been tested and have first-hand knowledge of the pleasantries of being swabbed to gather a sample. Although this article is not about testing for COVID-19, it does touch on similar concepts and procedures for environmental sampling that may be useful when performing assessments and evaluations to determine the current condition of a building.
The use of testing to verify present conditions of a project or to determine the effectiveness of a completed project for the restoration- and remediation-related industries can vary significantly. The key things to ask yourself when doing any type of testing are:
- What is the purpose of the testing?
- Is this testing meant to diagnose a problem or assist in adding additional information about a known condition within a building, or perhaps does the customer just want additional information on surfaces or air quality within his or her property?
Often, testing can be overapplied, and the results can be poorly interpreted or misinterpreted, resulting in conclusions that are simply not appropriate. Those conclusions can then drive certain recommendations that might not be warranted for a particular project.
This article’s purpose is not to explore the logic or decision-making of interpreting results; rather, it provides a very brief explanation of some of the typical methods and products used within the remediation and restoration industries so that understanding the usefulness and limitations of testing are better understood.
Environmental testing of any kind is primarily intended to provide general information and trend data as a representation of the environment being tested. There are exceptions to this, as in almost all cases, when a more diagnostic approach may be necessary in situations associated with medical or healthcare-related facilities, health and safety considerations, or in manufacturing of various products that require a high level of quality assurance and quality control. The representation of testing data can be applied to surfaces including finished building surfaces, building structural surfaces, or even contents. The other could include air quality testing for the evaluation of Condition 2 settled spores as defined by the ANSI/IICRC Standard for Professional Mold Remediation or to verify if microbial growth in a particular area or from a particular building material has resulted in the sporulation of contaminants into areas where this is undesirable. Sampling can be used to assist in confirming the presence of microbial growth whether it is mold, bacteria, or even viruses.
The following describes examples of basic types of testing materials, methods, and interpretation often used by indoor environmental professionals (IEPs), which could include industrial hygienists, mold assessors, home inspectors, and related professionals. It is important to note that this is not an exhaustive list, and combinations of a variety of different types of testing methods—including direct microscopy and spore trap or non-cultural testing, along with culturable testing—may be appropriate in various situations.
There are a variety of reasons for collecting and analyzing field samples as part of an assessment and investigation of indoor microbial contamination (which refers to fungal and/or bacterial contamination in the indoor environment for the purposes of this article). It becomes valuable to understand the why, how, and what to sample and test for. The key aspect to any sampling and analysis is to interpret the results as a part of the evaluation and assessment process and use this data in correlation with other field-gathered environmental data and observations.
Sampling and testing
There are no established governmental or professional protocol standards for sampling and testing of microbes. Best practices or procedures outlined by organizations such as the American Industrial Hygiene Association, American Conference of Governmental Industrial Hygienists, OSHA, etc., do exist and describe acceptable “traditional” sampling and analytical procedures. Many of the field methods used in microbial assessment and analyses are conventional or modified methods that are acceptable and used by microbiologists. Furthermore, there are no numeric health and safety standards (such as PEL or TLV) for airborne or surface microbial values for indoor assessment. General results interpretation is based on comparison of outdoor background, indoor references, or published interpretation data compiled by labs and similar organizations.
Some key concerns of sampling and testing include the following:
- One of the most overlooked and underappreciated considerations for performing sampling and testing is the selection of a laboratory. It is an extremely important factor in performing any testing and, like other items worth pursuing, requires some level of research to ensure a selected laboratory will provide you wth the information you need in the format that best fits your needs or your client’s needs.
- Once you have the lab selected, it is critical to select the most appropriate type of testing to sample the conditions of the environment. Some of the most common methods used are surface or air culturable fungi and bacteria, surface or air fungal spore/matter confirmation, and identification based on optical microscopy—or some variations of these.
- Determining the sampling equipment and supplies needed to implement the method(s) selected is an important aspect of testing that should not be undervalued.
Spore count/spore trap testing
This analysis is based on microscopic identification and enumeration of fungal spores and is only feasible for spore-trap air. Appropriate fungal identification is based on colony morphology or physical characteristics, spore production, and spore formation. The identification of the mold types based on spore alone is presumptive at best, and the viability or culturability of spores is unknown. Additionally, there is no standard or generally accepted analytical method, and it should be used or considered only as a screening tool.
However, in restoration or mold remediation situations, spore-count analysis provides a quick turnaround for the determination whether the remediation is cleared or not. This analysis is not suitable for samples collected from a dusty or dirty environment due to excessive dust overload and interference in the analysis identification and enumeration.
Benefits
- Useful for initial site testing, especially if fungal growth is not visible
- Quick and simple procedures and equipment
- Fast turnaround times available
- Low chance of sample contamination
Disadvantages
- Fungi cannot be fully speciated with this method. For example, Aspergillus sp. and Penicillium sp. are normally reported together due to the similarities in spore morphology (i.e., Asp/Pen).
- Spore viability cannot be assessed.
Basic procedure
The spore trap is actually a type of filter cassette designed to operate at a recommended flow rate of 15 lpm. Lower flow rates may result in a loss of some spores and the accumulation of others in a non-uniform manner. Higher flow rates may damage the spores. Therefore, it is important not to run the sampling pumps at lower flow rates for longer times to achieve the recommended air volume. Operate the sampling pump and sample for an appropriate period of time, typically 1-10 minutes depending on the total volumes desired:
- Clean “office” or outdoors (no visible dust) = 10 minutes
- Indoor environment, high activity and personnel = 5 minutes
- Indoor environment, drywall renovation or heavy industrial dust = 1 minute
Direct microscopy (tape lift, bulk, and swab)
Fungal confirmation and identification based on optical microscopy: Bulk or tape lift samples taken from visible, suspect “fungal growth” are analyzed microscopically for direct examination. Direct exam allows for the rapid determination of the presence of fungal spores and identifies the types of fungi. Direct examinations should only be used to sample visible mold growth in a contaminated area since most surfaces will have a deposited amount of fungal spores that are normally present in the environment.
This analysis is designed to confirm the observed suspect fungal growth and to identify observed fungi. This is a commonly used method in mold assessment. However, the interpretation of this type of testing is often misapplied for detecting and counting fungal spores from a surface with no sign of fungal growth. This testing is intended to identify mold organism type (genus level) present on a sampled surface rather than quantifying the numerical concentrations present.
Benefits
- Inexpensive and performed quickly
- A useful test for initial site sampling
- May reveal indoor reservoirs of spores that have not yet become airborne
Disadvantages
- Areas of fungal growth are often small and scattered, so they may not all be picked up. Multiple sampling will help overcome this problem.
- Health problems related to indoor microbial growth are generally caused by the inhalation of substantial numbers of airborne spores, sometimes over a long period of time. The presence of biological materials on a particular surface may not be a direct indication of what is in the air.
- This method detects both viable and non-viable spores but cannot distinguish between them. It is advisable to combine direct exam samples with culture methods if knowing viability is important to your project.
- Tape lifts are not able to be cultured. If a direct examination of a swab sample is taken, a followup by culture is possible.
- Direct examinations of dirt/soil and dust samples cannot be performed reliably.
- Fungi usually cannot be identified to species and sometimes not even to genus with this method. For example, Aspergillus sp. and Penicillium sp. are normally reported together due to the similarities in spore morphology, unless fruiting structures are present that allows for a better identification.
Materials needed for sample collection and transport
- Tape lift:
- Biotapes (brand type)
- Clear (transparent) Scotch or other brand tape (Frosted tape obscures spores.)
- New plastic bag to hold specimen(s)
- For bulk: Sterile container—new plastic bag or Whirl-a Pack (provided at your request by lab) to hold and transport specimen
- For swab: Sterile culturette/swab with appropriate buffer solution to collect and transport specimen (provided at your request by lab)
- For all matrices: Latex/nitrile gloves
Culturable testing (air, surface, swab, etc.)
This analysis generally requires similar collections methods as the spore trap and microscopy sampling and testing. The samples have to go through an incubation period of at least three to seven days on appropriate nutrient media and incubation temperature ranges. Samples can be air, wipe, bulk, dust, or liquid. For general purposes, incubation at near 25 degrees Celcius (77 degrees Fahrenheit) for at least three to seven days is suggested. If samples are collected from healthcare facilities and opportunistic fungal pathogens (such as Aspergillus fumigatus) are of concern, samples should be incubated at 35-37 degrees Celcius (95-98.6 degrees Fahrenheit) for two days or longer. Fungal nutrient media may be for general isolation or for selective isolation.
General purpose media include malt extract agar (MEA) and cornmeal agar (CMA) are sometimes used in the description of fungi, for instance, CMA used in describing species of Stachybotrys and Memnoniella in culture. MEA is important in studies of Aspergillus and Penicillium in culture and is a common medium used by mycologists. These methods are best for specific identification and enumeration of fungi or bacteria; however, due to the significant time it takes to incubate samples for adequate analysis for three to seven days, these general methods are not often used for restoration- and remediation-related projects that require fast turnaround times (less than 48 hours).
Results interpretation and quality control (fungal air testing)
An effective interpretation of air samples is generally based on the comparison of indoor and outdoor samples. An outdoor sample will help demonstrate whether spore amplification is occurring indoors. Another “control” sample can be obtained from an indoor, non-compliant sample area for comparison as well. Both methods of control samples can be useful and may be included in a set of project assessment samples depending on the scope of the project. Because there are no generally accepted guidelines or standards for fungal bioaerosols, comparative sampling becomes necessary.
Usually, outdoor samples and samples taken from non-problem locations are collected as a reference for comparison to the “investigation” samples. A few proposed guidelines for fungi have been published; however, they should be used with care and only for screening purposes—and not as a “safe” or “unsafe” health standard or for medical diagnosis. These guidelines may also be considered as “performance standards.” The importance of reference samples is such that a significant number should be collected for each job.
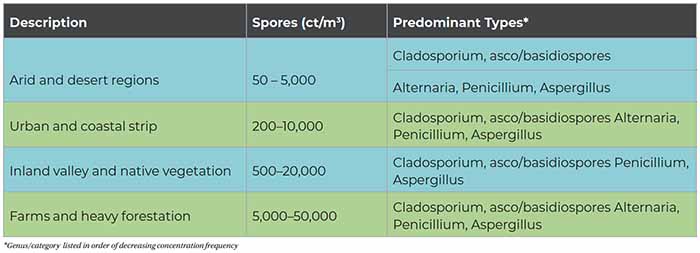
For practical purposes, comparisons with reference samples are the most useful approach. In general, indoor fungal concentrations should be similar to or lower than outdoors. Residential buildings tend to have fungal levels similar to outdoors. Indoor fungal types should also be similar to outdoors.
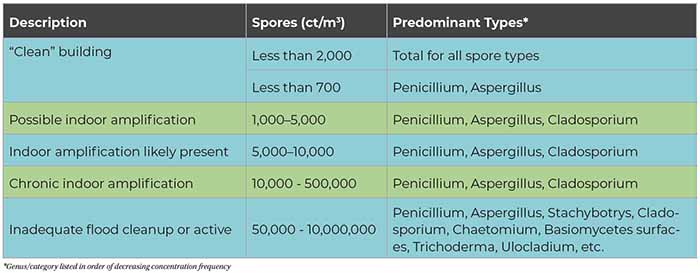
If fungi at a significant level are only found indoors, this often suggests indoor amplification or growth of the fungi due to excess water incursion or water damage to building materials or contents. Furthermore, the detection of some fungi even at low levels in the air may require further evaluation. Any presence of toxigenic fungi, such as Stachybotrys chartarum and Acremonium strictum, may suggest an indoor contamination source and should be investigated further.
However, the presence of these fungi at trace levels can be expected and does not mean there is fungal growth amplification. The consistent detection of some fungi, such as species of Aspergillus, Paecilomyces, Penicillium, Trichoderma, and Wallemia in multiple sample locations or between sample types (air and surface) could indicate water damage and fungal amplification of building materials or contents.
A high variability in outdoor mold spore concentrations and distribution exists on a daily to hourly basis and is dependent on local vegetation and micro-climate, the time of year, local weather patterns, and diurnal variation. As a result, caution must be used when simultaneously comparing limited data sets of inside and outside concentrations or overgeneralizing any set of data to conditions in desert or snow-covered environments.
Tables 1 and 2 can serve as a rough guide to evaluating the relative degree of potential indoor airborne mold spore amplification. The term “clean” refers to the classifcation definition of buildings given in the AIHA 2005 publication A Regional Comparison of Mold Spore Concentrations Outdoors and Inside “Clean” and “Mold Contaminated Southern California Buildings. “Clean” refers to a building found to have no current evidence of historical water intrusion and no visible evidence of elevated moisture conditions or mold growth determined by a systematic and thorough visual inspection.
As a general rule, total indoor airborne spore concentrations in a typical “clean” HVAC-supplied building are less than the “average” regional outside concentrations and/or less than approximately 1,000 cts/m3. Organism types such as Aspergillus and Penicillium and other hyaline spores are on average less than 700 cts/m3. Indicator fungi such as Stachybotrys, Chaetomium, and Ulocladium are often recovered in low concentrations in indoor samples as a result of normal infiltration; therefore, automatically assuming there is indoor growth when low concentrations of any indicator species are detected is inappropriate and could provide for inaccurate interpretation of the actual conditions.
Remember, there is always a likely exception to every rule or generalization, and because there is no direct relationship between simultaneously collected indoor and outdoor samples, performing a direct comparison with limited sampling is often misleading. The range of expected variability could have differences of 5-10 fold when comparing limited data sets, and this too must be considered.
Final thoughts on environmental testing
The use of sampling or testing in combination with a thorough and comprehensive assessment and inspection can provide significant value for restoration and remediation projects. The types of sampling performed (air, surface, etc.) in the field will depend on the goal of the project and conditions present within a building. Additionally, the use of spore trap or direct microscopy analysis versus culture sampling and analysis will depend on the project requirements.
Projects that demand fast turnaround time for results (24 hours or so) should opt for the non-culturable analysis, and those projects that may involve understanding the potential for occupant exposure to potentially infectious agents or pathogens may want to implement culture testing—understanding this analysis may take three to seven days. Furthermore, the interpretation of any laboratory data can vary greatly on the criteria for restoration and remediation performance, geographic location of the project, weather conditions, etc., so a uniform standard for interpretation is difficult to apply globally and the resulting conclusions for the data should be thoughtfully considered by trained professionals.
Samples and testing are meant to represent the conditions present in the air and on surfaces within the indoor environment. No matter what the conditions are or types of testing used on a project, the most important consideration is to understand the correct applications and limitations of any testing and to not rely on it as a defining diagnostic tool that could potentially bias a conclusion of performance or lack thereof.
Slade K. Smith, RPIH, AIEH, is an IICRC master restorer, a registered professional industrial hygienist, and is the owner, president, and CEO of the Industrial Hygiene Consulting Corporation, a private environmental consulting and testing firm focused on identifying and solving indoor environmental problems, building-related deficiencies, disaster and water loss consulting, and microbial impacts of water loss events. He can be reached via email at [email protected].
References
- Yang, C. S., 2004, Assessment of Fungal Contamination in Buildings, Aerotech P&K Microbiology Services, Inc., Cherry Hill, New Jersey, Version 2003-1
- Dillon, H. K., P. A. Heinsohn, and J. D. Miller. 1996. Field Guide for the Determination of Biological Contaminants in Environmental Samples. AIHA Publications, Fairfax, VA.
- Buttner, M.P. & L.D. Stentzenbach. 1993. Monitoring airborne fungal spores in an experimental indoor environment to evaluate sampling methods and the effects of human activity on air sampling. Appl. & Env. Microbiology, 59: 219- 226.
- Johanning, E. and C. S. Yang. 1995. Fungi and Bacteria in Indoor Air Environments, Health Effects, Detection and Remediation, the Proceeding of the International Conference on Fungi and Bacteria in Indoor Air Environments, October 6-7, 1994. Eastern New York Occupational Health Program, Albany, New York.
- Yang, C. S., L-L Hung, F. A. Lewis, and F. A. Zampiello. 1993. Airborne fungal populations in non-residential buildings in the United States. Proceedings of Indoor Air ’93, 4: 219-224. Helsinki, Finland.
- Stevens, R. B. 1981. Mycology Guidebook. 712 pp. University of Washington Press, Seattle.
- Jong, S. C. and E. E. Davis. 1976. Contribution to the knowledge of Stachybotrys and Memnoniella in culture. Mycotaxon 3: 409-485.
- Pitt, J. 2000. A Laboratory Guide to Common Penicillium species. 197 pp. Food Science Australia, NSW, Australia.
- Klich, M. A. and J. I. Pitt. 1988. A Laboratory Guide to Common Aspergillus and their Teleomorphs. 116 pp. CSIRO. NSW, Australia.
- EMSL Analytical, Inc., 2006, Microbiology Sampling Guide., Westmont, NJ
- Baxter, Daniel M., Air-O-Cell Method Guide and Particle Atlas, 2-7 pp., Environmental Analysis Associates, 2013, San Diego, CA