Eliminating Odors

There is something new in eliminating odors and sanitizing—old technology with a profound upgrade. That methodology is oxidation. The chemistry of oxidizers can destroy odors as well as stains.
Newer oxidizers
Most experienced cleaners are familiar with oxides such as 3% hydrogen peroxide, sodium percarbonate boosters for cleaning, spotters with the word “stain” in the name, and ozone plus hydroxyl generators. These newer oxidizers are made from substances like chlorine dioxide, peracetic acid, and other substances, plus ultraviolet-C (UVC) light.
Comparing the abilities of these newer oxidizers to common ones of the past is like comparing older hydrofluoric acid rust removers to more recent and safer products. The 20% hydrofluoric acid rust removers of the past worked in a split second but created a substantial risk for the untrained user. Because technicians were ignorant of the hazards, suppliers chose safety over performance. This meant that cleaners lost a product that worked quickly for one that had a lesser ability to get the job done. Hopefully, this doesn’t happen with the new, advanced forms of oxidation, which have profoundly impacted the cleaning industry.
Understanding oxidizers

This device directs the UVC light down to protect the user and focus the energy onto the floor.
To understand these newer types of oxidizers, practical knowledge of a numerical system to describe their strength is necessary. For comparison, the pH scale is used to understand the strength of acids and alkalines. Acids are proton donors, while alkalines are proton receivers. The proton is noted as H+. This gives acids a positive charge while alkalines receive a negative one.
However, atoms are made up of two charged particles that create a propensity for chemical reactions. The second charged particle is the electron. This is the chemistry of bleach, which comes in two forms—reducers and oxidizers. In the cleaning industry, it has been said that the reducers remove oxygen while oxidizers add it. While that is frequently true, it is really about electrons instead of oxygen. The system that chemists use for oxidizers and reducers is called the redox scale. Since we are talking about electrons, redox propensities are measured in millivolts, i.e., 1 volt = 1,000 millivolts, whose acronym is “mv.”
Here are popular examples of oxidation-reduction potential (ORP) values of different substances that should be familiar to most cleaners.
Hydrogen peroxide (H2O2), at pharmaceutical strength, is a 3% solution and with an ORP of 360 mv. As a stain remover, it could destroy coffee, tea, or cellulosic browning stains. In similar comparisons, it would have a mild effect on urine or biological odors. Sodium percarbonate also has an ORP strength of 360 mv. In other words, 360 mv does not require special training to be used properly.
Strong dye stain removers have ORP values from 650 to 850 mv. Lack of training can lead to bodily and material damage.
The newer oxidizers for deodorizing are from 900 to 2,000+. In part, this explains why they can do things that couldn’t be achieved in the past.
Whereas ORP values could be compared to pH values for understanding reducers and oxidizers—and while three editions of the IICRC S100 state that a pH value is only one of different measurements of the strength of an acid or alkaline and that it alone is an inadequate value in knowing its effects on fibers—ORP values are similar as well. As with pH, one should realize anyone who teaches that pH or ORP is the measurement of either is doing a disservice to the understanding of either topic. Thus, to appreciate an ORP value, trainers need to correct years of misinformation.
Newer forms of oxidation
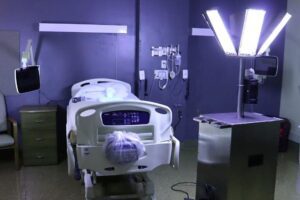
This device, typically used in hospital patient rooms, increased inspection ratings from the low seventies into the nineties in a fraction of the time it took to manually clean the room. (Photos courtesy of Larry Cobb)
The newer forms of oxidation have played a significant role in fighting the pandemic, but it has not come entirely in the form of a liquid packaged in a bottle. The U.S. Environmental Protection Agency (EPA) has recognized UVC light’s ability to destroy organisms. This type of ultraviolet light, in the “C” bandwidth, is not your typical black light for inspections and stain removal. These lights cost thousands of dollars and can generate substantial revenue for the operator who knows where and how to use them.
These kinds of lights have hazards as well. They can damage eyes. When used during the COVID-19 pandemic, hospital rooms had to be vacated. The financial rewards were substantial for the lucky few who knew of this groundbreaking approach. What is the answer? While there is something new that can change our industry by broadening its opportunities, we need training. The questions to be answer are such: Who will do that training, and will their understanding of redox and UVC be adequate to make this method safe, effective, and inclusive?












